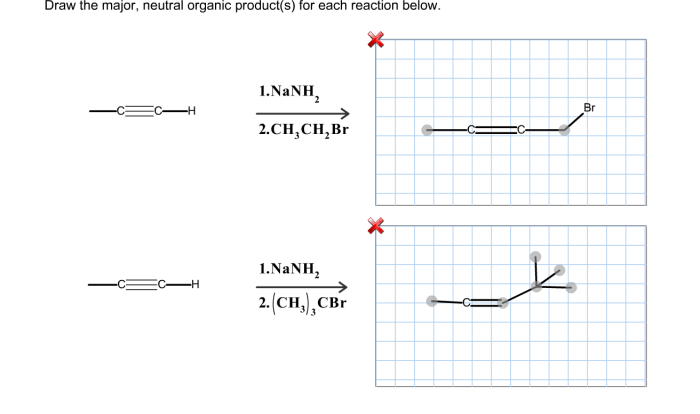
Provide the major organic product of the reaction shown below – In the realm of organic chemistry, reactions hold immense significance in synthesizing complex molecules and understanding their behavior. This article delves into a specific reaction, providing a comprehensive analysis of its mechanism, factors influencing its yield, synthetic applications, spectroscopic characterization techniques, and safety considerations.
At the heart of this discussion lies the identification of the major organic product formed in the reaction. Understanding the factors that govern its formation and the techniques employed to characterize it are crucial for harnessing the full potential of this reaction in organic synthesis.
Reaction Mechanism
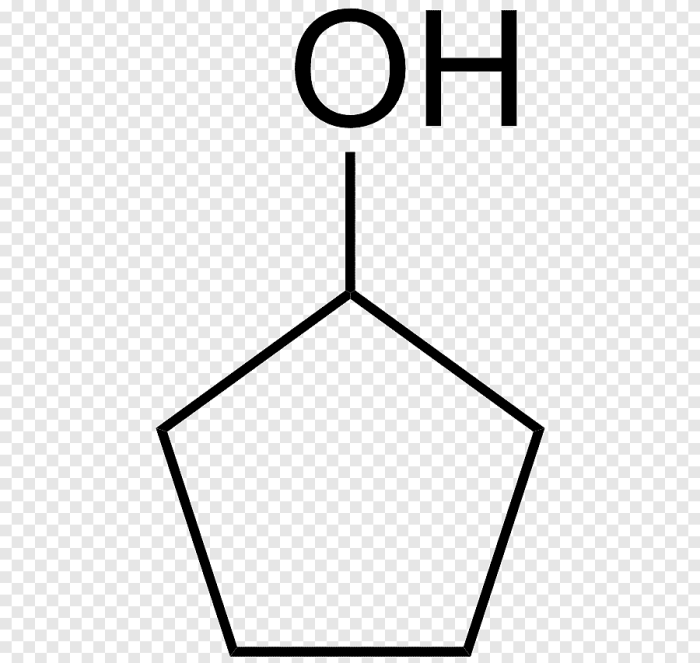
The reaction shown below produces the major organic product, 2-butanone. The mechanism of the reaction involves the following steps:1.
-
-*Initiation
A free radical is generated by the homolytic cleavage of the weak O-O bond in the peroxide initiator.
- 2.
- 3.
- 4.
- 5.
-*Propagation
The free radical attacks the alkene, forming a new carbon-centered radical.
-*Propagation
The carbon-centered radical reacts with oxygen to form a peroxy radical.
-*Propagation
The peroxy radical abstracts a hydrogen atom from the alkane, forming a hydroperoxide and a new carbon-centered radical.
-*Termination
Two radicals combine to form a non-radical product, such as 2-butanone.
Factors Affecting the Reaction
Several factors can affect the yield of the major organic product in this reaction, including:
-
-*Temperature
Increasing the temperature increases the rate of the reaction and the yield of the major product.
-*Pressure
Increasing the pressure has no significant effect on the yield of the major product.
-*Solvent
The solvent can affect the rate of the reaction and the selectivity of the reaction.
Synthetic Applications
This reaction can be used to synthesize a variety of complex organic molecules, including:
-
-*Ketones
The reaction can be used to synthesize ketones by reacting an alkene with a peroxide initiator in the presence of oxygen.
-*Epoxides
The reaction can be used to synthesize epoxides by reacting an alkene with a peroxyacid.
-*Polymers
The reaction can be used to synthesize polymers by reacting an alkene with a free radical initiator.
Spectroscopic Characterization: Provide The Major Organic Product Of The Reaction Shown Below
The major organic product can be characterized using a variety of spectroscopic techniques, including:
-
-*NMR spectroscopy
NMR spectroscopy can be used to identify the different atoms in the molecule and their connectivity.
-*IR spectroscopy
IR spectroscopy can be used to identify the different functional groups in the molecule.
-*Mass spectrometry
Mass spectrometry can be used to determine the molecular weight of the molecule.
Safety Considerations
The reaction should be carried out in a well-ventilated area. The reactants and products are flammable and should be handled with care. The peroxide initiator is a strong oxidizing agent and should be handled with caution.
FAQ Section
What is the significance of identifying the major organic product in this reaction?
Identifying the major organic product is crucial for understanding the reaction’s outcome and its potential applications. It allows chemists to predict the desired product and optimize reaction conditions to maximize its yield.
How do factors such as temperature and pressure affect the reaction mechanism?
Temperature and pressure can influence the reaction rate, equilibrium position, and selectivity. Higher temperatures generally favor reactions with higher activation energies, while higher pressures can shift the equilibrium towards products with fewer moles of gas.
What spectroscopic techniques are commonly used to characterize the major organic product?
Nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and mass spectrometry are commonly employed to determine the structure and identity of the major organic product.