Reading the heating/cooling curve homework – Embarking on a journey through the intricacies of heating/cooling curves, this comprehensive guide unravels the fundamental principles, applications, and advanced techniques involved in this indispensable tool for material characterization.
Delving into the intricacies of heating/cooling curves, this guide equips readers with a comprehensive understanding of their purpose, interpretation, and practical applications.
Understanding Heating/Cooling Curves
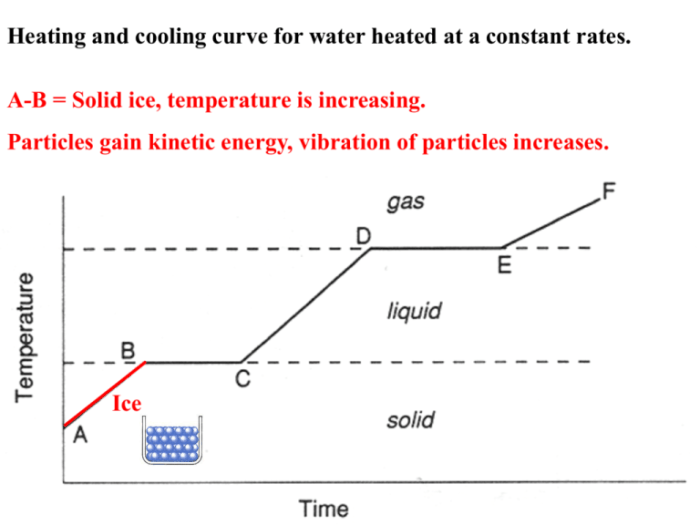
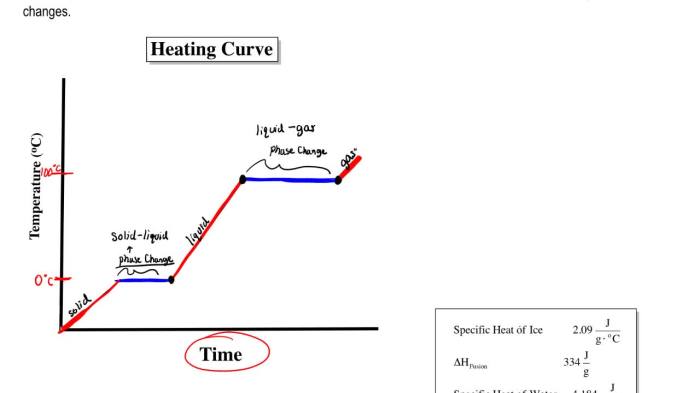
Heating/cooling curves are graphical representations of the temperature changes that occur in a substance as it is heated or cooled. They provide valuable information about the thermal properties of materials and are widely used in various fields of science and engineering.
The basic principle of thermal analysis is to measure the temperature of a sample as it undergoes a controlled heating or cooling process. By monitoring the temperature changes, it is possible to identify thermal transitions such as melting, freezing, crystallization, and phase changes.
There are different types of heating/cooling curves, each with its own characteristic features. The most common types include:
- Melting curve: Shows the temperature at which a solid substance melts and transitions into a liquid state.
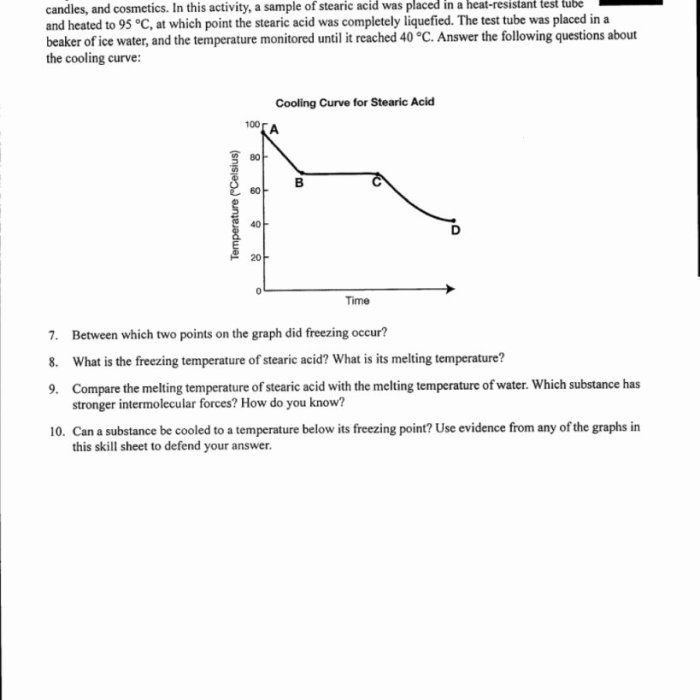
- Freezing curve: Shows the temperature at which a liquid substance freezes and solidifies.
- Crystallization curve: Shows the temperature at which a liquid substance crystallizes and forms a solid structure.
- Phase transition curve: Shows the temperature at which a substance undergoes a change in its physical state, such as from solid to liquid or liquid to gas.
Interpreting Heating/Cooling Curves
Heating/cooling curves provide valuable information about the thermal properties of materials. By analyzing the characteristic features of these curves, it is possible to determine the following:
- Melting point: The temperature at which a solid substance melts and transitions into a liquid state.
- Freezing point: The temperature at which a liquid substance freezes and solidifies.
- Other thermal transitions: Such as crystallization, phase changes, and glass transitions.
The shape and appearance of heating/cooling curves can be affected by various factors, including:
- The rate of heating or cooling
- The sample size and shape
- The presence of impurities or defects
- The thermal conductivity of the material
Applications of Heating/Cooling Curves
Heating/cooling curves are widely used in various fields, including:
- Material characterization: To determine the thermal properties of materials, such as melting point, freezing point, and phase transition temperatures.
- Polymer science: To study the thermal behavior of polymers, including their melting, crystallization, and glass transition temperatures.
- Pharmaceuticals: To determine the melting point and other thermal properties of drugs and pharmaceutical compounds.
- Food science: To study the thermal behavior of food products, such as their melting, freezing, and crystallization temperatures.
- Metallurgy: To study the thermal properties of metals and alloys, including their melting point, freezing point, and phase transition temperatures.
Heating/cooling curves offer several advantages for material characterization, including:
- Simplicity: They are relatively simple and straightforward to perform.
- Versatility: They can be used to study a wide range of materials.
- Sensitivity: They can detect small changes in thermal properties.
Advanced Analysis of Heating/Cooling Curves, Reading the heating/cooling curve homework
In addition to basic interpretation, advanced techniques can be employed to analyze heating/cooling curves in more detail.
Differential scanning calorimetry (DSC) is a powerful technique that measures the heat flow into or out of a sample as it undergoes a controlled heating or cooling process. DSC curves provide detailed information about the thermal transitions that occur in the sample, including their enthalpy and temperature.
Other advanced techniques include:
- Modulated temperature differential scanning calorimetry (MTDSC)
- Thermogravimetric analysis (TGA)
- Dynamic mechanical analysis (DMA)
These techniques provide complementary information to heating/cooling curves and can be used to obtain a more comprehensive understanding of the thermal properties of materials.
Visualizing Heating/Cooling Curves
| Type of Curve | Key Features |
|---|---|
| Melting curve | Shows the temperature at which a solid substance melts and transitions into a liquid state. |
| Freezing curve | Shows the temperature at which a liquid substance freezes and solidifies. |
| Crystallization curve | Shows the temperature at which a liquid substance crystallizes and forms a solid structure. |
| Phase transition curve | Shows the temperature at which a substance undergoes a change in its physical state, such as from solid to liquid or liquid to gas. |
Flowchart Illustrating the Process of Interpreting Heating/Cooling Curves
- Identify the characteristic features of the curve.
- Determine the melting point, freezing point, and other thermal transitions.
- Consider the factors that can affect the shape and appearance of the curve.
- Interpret the results and draw conclusions about the thermal properties of the material.
Key Questions Answered: Reading The Heating/cooling Curve Homework
What are the key features of heating/cooling curves?
Heating/cooling curves exhibit characteristic features such as melting points, freezing points, and other thermal transitions, providing insights into the thermal behavior of materials.
How can heating/cooling curves be used for material characterization?
Heating/cooling curves offer valuable information about a material’s thermal properties, phase transitions, and purity, aiding in material selection and optimization.
What are the limitations of using heating/cooling curves?
Heating/cooling curves may be affected by factors such as sample size, heating/cooling rate, and equipment sensitivity, which can impact the accuracy and interpretation of the results.