Embark on a scientific exploration with Lab 1 Diffusion and Osmosis Answers, a comprehensive guide that unveils the fundamental principles governing the movement of molecules and fluids across biological membranes. Delve into the fascinating world of diffusion and osmosis, exploring their essential roles in everyday life, biological systems, and cutting-edge applications.
From the intricate processes within plant and animal cells to their practical implications in medicine and industry, this guide provides a thorough understanding of these vital concepts.
Diffusion
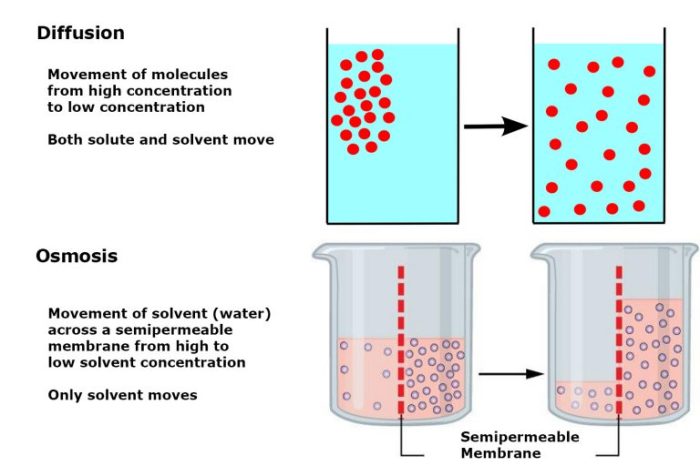
Diffusion is the net movement of molecules from an area of high concentration to an area of low concentration.
Diffusion is a passive process, meaning that it does not require energy. It is driven by the concentration gradient, which is the difference in concentration between two areas.
Factors Affecting the Rate of Diffusion
The rate of diffusion is affected by several factors, including:
- Concentration gradient:The greater the concentration gradient, the faster the rate of diffusion.
- Temperature:The higher the temperature, the faster the rate of diffusion.
- Surface area:The greater the surface area, the faster the rate of diffusion.
- Distance:The shorter the distance between the two areas, the faster the rate of diffusion.
- Size of molecules:The smaller the molecules, the faster the rate of diffusion.
Examples of Diffusion in Everyday Life
Diffusion is a common phenomenon that occurs in many everyday situations, including:
- The spread of perfume in a room
- The diffusion of oxygen from the lungs to the blood
- The diffusion of carbon dioxide from the blood to the lungs
- The diffusion of nutrients from the small intestine to the blood
- The diffusion of waste products from the blood to the kidneys
Osmosis
Osmosis is the movement of water molecules across a semipermeable membrane from an area of high water concentration to an area of low water concentration. The semipermeable membrane allows water molecules to pass through but prevents the passage of solutes (dissolved particles).
Osmosis is a crucial process in biological systems, as it maintains the water balance and volume of cells. It also plays a role in the transport of nutrients and waste products across cell membranes.
Osmosis in Plant Cells
In plant cells, the cell wall provides support and prevents the cell from bursting when water enters. When a plant cell is placed in a hypotonic solution (a solution with a lower solute concentration than the cell), water moves into the cell by osmosis, causing the cell to swell and become turgid.
This turgidity provides support to the plant and helps it to stand upright.
Osmosis in Animal Cells
Animal cells do not have a cell wall, so they are more susceptible to changes in osmotic pressure. When an animal cell is placed in a hypotonic solution, water moves into the cell, causing the cell to swell and eventually burst.
This process is called cytolysis.
When an animal cell is placed in a hypertonic solution (a solution with a higher solute concentration than the cell), water moves out of the cell by osmosis, causing the cell to shrink and become crenated.
Importance of Osmosis in Biological Systems
Osmosis is essential for the proper functioning of biological systems. It plays a role in:
- Maintaining the water balance of cells
- Transporting nutrients and waste products across cell membranes
- Providing support to plant cells
- Regulating blood pressure
- Maintaining the fluid balance in the body
Lab 1 Diffusion and Osmosis
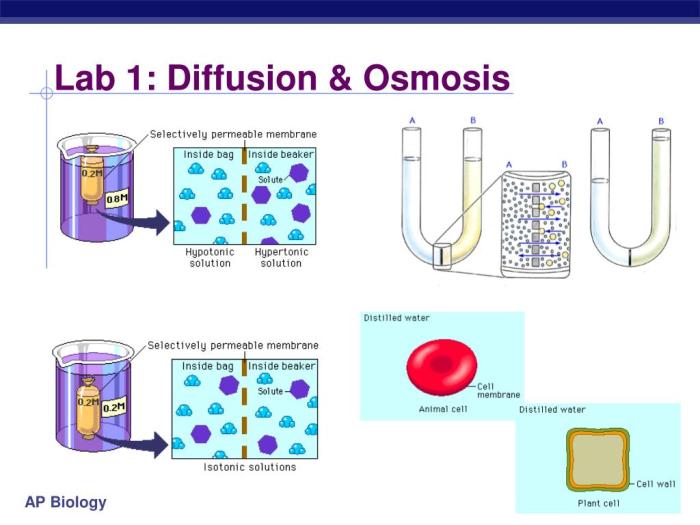
Lab 1 Diffusion and Osmosis explores the fundamental processes of diffusion and osmosis, which are critical to understanding the movement of molecules across biological membranes.
Experimental Setup and Procedures
The lab involves setting up two experimental setups:
- Diffusion setup: A semipermeable membrane separates two compartments, one containing a high concentration of a solute (e.g., glucose) and the other containing pure water.
- Osmosis setup: A semipermeable membrane separates two compartments, one containing a high concentration of a solute (e.g., salt) and the other containing pure water.
In both setups, the changes in solute concentration and water movement are observed and recorded over time.
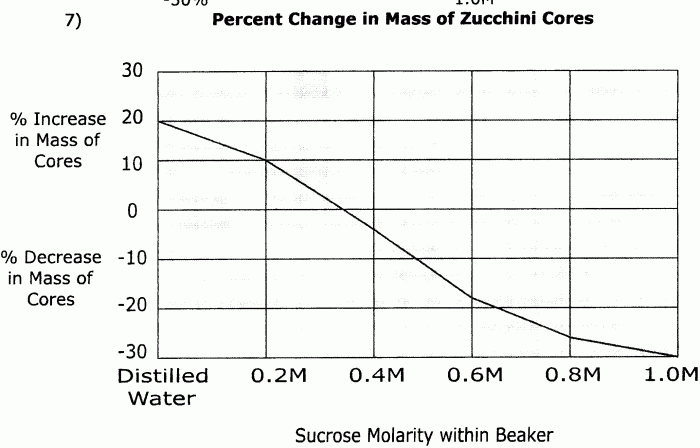
Observations and Results
- Diffusion: Molecules of the solute diffuse from the high-concentration compartment to the low-concentration compartment until equilibrium is reached, resulting in a gradual decrease in concentration in the high-concentration compartment and a corresponding increase in the low-concentration compartment.
- Osmosis: Water molecules move from the low-concentration compartment to the high-concentration compartment, causing the volume of the high-concentration compartment to increase and the volume of the low-concentration compartment to decrease.
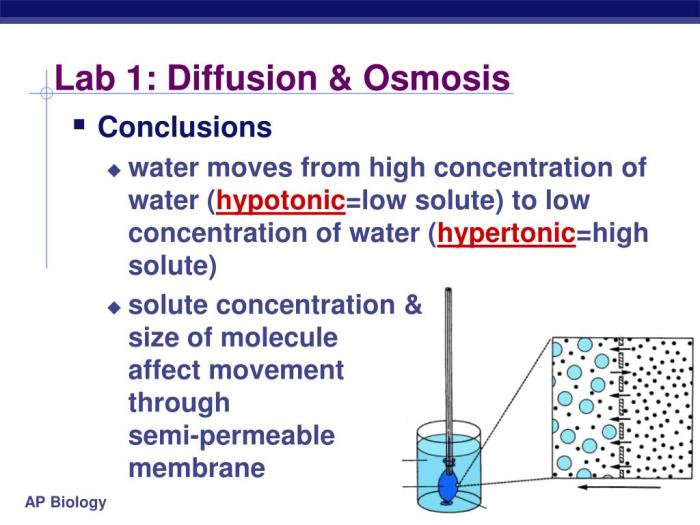
Conclusions
The lab results demonstrate that:
- Diffusion is the net movement of molecules from an area of high concentration to an area of low concentration, driven by the concentration gradient.
- Osmosis is the net movement of water molecules across a semipermeable membrane from an area of low solute concentration to an area of high solute concentration, driven by the osmotic pressure gradient.
These processes are essential for the transport of nutrients, waste products, and water in living organisms.
Applications of Diffusion and Osmosis
Diffusion and osmosis are fundamental processes that play crucial roles in various aspects of everyday life, medicine, and industry. These processes facilitate the movement of molecules across semipermeable membranes, driving essential functions in living organisms and enabling technological advancements.
Everyday Applications, Lab 1 diffusion and osmosis answers
* Respiration: Diffusion enables the exchange of oxygen and carbon dioxide in the lungs, allowing organisms to obtain vital oxygen and expel waste gases.
Nutrient Absorption
In the digestive system, diffusion transports nutrients from the intestines into the bloodstream for distribution throughout the body.
Waste Removal
Kidneys use osmosis to filter waste products from the blood and excrete them as urine.
Medical Applications
* Drug Delivery: Osmosis-based drug delivery systems release medication over time, providing sustained and targeted treatment.
Dialysis
Dialysis machines utilize diffusion to remove excess waste and fluids from the blood of patients with kidney failure.
Cell Culture
Diffusion is essential for cell culture, enabling the exchange of nutrients and oxygen with the surrounding medium.
Industrial Applications
* Water Purification: Reverse osmosis removes impurities and contaminants from water, producing clean and potable drinking water.
Food Preservation
Osmotic dehydration removes moisture from food, extending shelf life and preserving flavor.
Textile Manufacturing
Diffusion processes are used to dye and treat fabrics, imparting desired properties and enhancing their quality.
Potential Future Applications
* Tissue Engineering: Osmosis and diffusion could facilitate the development of artificial tissues and organs for transplantation.
Nanotechnology
Diffusion-based techniques may enable the precise delivery of nanoparticles for targeted drug delivery and imaging.
Environmental Remediation
Osmotic processes could be harnessed to remove pollutants from soil and water, improving environmental sustainability.
Helpful Answers: Lab 1 Diffusion And Osmosis Answers
What is the key difference between diffusion and osmosis?
Diffusion involves the movement of molecules from an area of high concentration to an area of low concentration, while osmosis specifically refers to the movement of water molecules across a semipermeable membrane from an area of low solute concentration to an area of high solute concentration.
How does osmosis play a crucial role in plant cells?
Osmosis maintains turgor pressure in plant cells, which is essential for their structural integrity and growth. Water moves into the cell when the external solution is hypotonic, causing the cell to swell and become turgid. Conversely, in a hypertonic solution, water moves out of the cell, leading to plasmolysis.
What are some practical applications of diffusion and osmosis in medicine?
Diffusion and osmosis principles underlie various medical applications, such as drug delivery systems, dialysis, and artificial respiration. Understanding these processes enables the development of innovative treatments and technologies to improve patient outcomes.
